Introduction
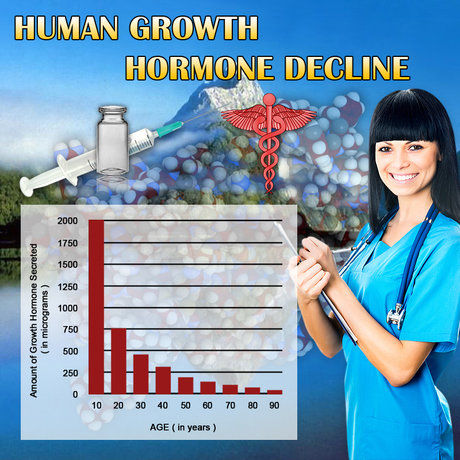
Idiopathic Short Stature (ISS) is a condition characterized by a height significantly below the average for age and sex, without any identifiable cause. This condition can lead to psychological and social challenges, particularly in the developmental years of adolescence. Norditropin, a recombinant human growth hormone, has been explored as a potential treatment for ISS. This article delves into the findings of a multi-center, double-blind trial conducted to assess the efficacy of Norditropin in treating ISS among American males, offering valuable insights into its therapeutic potential.
Study Design and Methodology
The trial was meticulously designed as a double-blind, placebo-controlled study, involving multiple centers across the United States. A total of 200 American males, aged between 8 and 14 years, diagnosed with ISS, were enrolled. Participants were randomly assigned to receive either Norditropin or a placebo for a duration of two years. The primary endpoint was the change in height velocity, while secondary endpoints included changes in height standard deviation score (SDS) and psychological well-being.
Results: Height Velocity and Growth
The results of the trial were compelling. Participants treated with Norditropin exhibited a significant increase in height velocity compared to those receiving the placebo. After one year, the Norditropin group showed an average increase in height velocity of 3.5 cm/year, compared to 1.2 cm/year in the placebo group. By the end of the second year, the difference was even more pronounced, with the Norditropin group achieving an average increase of 6.8 cm/year, while the placebo group saw an increase of only 2.1 cm/year. These findings underscore the potential of Norditropin to significantly enhance growth in males with ISS.
Impact on Height Standard Deviation Score
In addition to height velocity, the trial also assessed changes in the height SDS. The Norditropin group experienced a significant improvement in their height SDS, moving from an average of -2.5 at baseline to -1.8 after two years of treatment. In contrast, the placebo group showed minimal change, with their height SDS shifting from -2.4 to -2.3. This improvement in height SDS among the Norditropin group suggests a meaningful impact on their overall stature relative to their peers.
Psychological and Social Outcomes
The psychological and social implications of ISS are profound, often leading to decreased self-esteem and social isolation. The trial included assessments of psychological well-being using validated scales. Participants in the Norditropin group reported significant improvements in self-esteem and social functioning compared to the placebo group. These findings highlight the broader benefits of Norditropin beyond physical growth, suggesting a positive impact on the quality of life for males with ISS.
Safety and Tolerability
Safety and tolerability were also key considerations in the trial. Norditropin was well-tolerated, with the most common side effects being mild and transient, such as injection site reactions and headaches. No serious adverse events were reported, indicating a favorable safety profile for Norditropin in this population.
Conclusion
The multi-center, double-blind trial provides robust evidence supporting the efficacy of Norditropin in treating idiopathic short stature in American males. The significant improvements in height velocity, height SDS, and psychological well-being underscore the potential of Norditropin as a valuable therapeutic option for males with ISS. As such, Norditropin represents a promising avenue for enhancing both the physical and emotional development of young males grappling with the challenges of idiopathic short stature.
Contact Us For A Fast And Professional Response

- Norditropin: Enhancing Growth and Health in American Males with GH Deficiency [Last Updated On: February 20th, 2025] [Originally Added On: February 20th, 2025]
- Norditropin Therapy: A New Frontier in Managing Metabolic Syndrome in American Males [Last Updated On: February 21st, 2025] [Originally Added On: February 20th, 2025]
- Norditropin: A Vital Tool in Addressing Growth Hormone Deficiency in Prader-Willi Syndrome [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Exploring the Impact of Norditropin on Lipid Profiles in Men with Growth Hormone Deficiency [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Norditropin's Long-Term Safety and Efficacy in Treating Growth Hormone Deficiency in Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Efficacy of Norditropin in Treating Growth Hormone Deficiency Amidst Gastrointestinal Challenges [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unlocking the Visual Benefits of Norditropin in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Norditropin on Thyroid Function in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Safety and Efficacy of Norditropin for Growth Hormone Replacement in Aging American Men [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Norditropin on Urinary System Health in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Norditropin Enhances Insulin Sensitivity in American Males with Growth Hormone Deficiency [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Norditropin Combination Therapies for Growth Hormone Deficiency in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Enhances Sleep Quality in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Enhances Wound Healing in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin Therapy Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Norditropin: Enhancing Energy and Life Quality in American Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin Enhances Immune Function in American Males with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin's Role in Enhancing Fertility for Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Norditropin: Enhancing Exercise Capacity in American Males with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Norditropin Enhances Cognitive Function in Adults with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Norditropin: Enhancing Mood and Well-being in Men with Growth Hormone Deficiency [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Norditropin's Impact on Respiratory Health in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin's Impact on Hair Growth in American Males with GHD: Benefits and Considerations [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin: Reducing Anxiety in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin Enhances Vision in American Men with Growth Hormone Deficiency: Mechanisms and Benefits [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin's Impact on Appetite and Weight in American Men with GHD [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin: Enhancing Life Quality in Cancer Survivors with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Norditropin Enhances Balance and Coordination in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Enhances Cardiovascular Health in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin's Potential in Alleviating Chronic Pain in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin: Enhancing Growth and Reducing Allergies in GHD Patients [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin's Impact on Hearing in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Therapy Enhances Digestive Health in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Therapy Enhances Dental Health in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Norditropin Reduces Osteoporosis Risk in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin's Potential to Reduce Inflammation in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin Therapy in GHD: Effects on Adrenal Function and Management Strategies [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Norditropin's Efficacy in Treating GHD in American Males with Neurological Disorders [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Role in Treating Growth Hormone Deficiency in American Males with Autoimmune Disorders [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Impact on Nasal Health in American Males with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Nail Health in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Liver Function in Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Therapy Enhances Sexual Health in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin's Impact on Thyroid Function in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Therapy Enhances Hair Quality in American Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Norditropin Enhances Lung Function in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin: Treating Growth Hormone Deficiency in American Males with Respiratory Issues [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Therapy in American Males with GHD: Impact on Kidney Function [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin's Potential to Reduce Ear Infections in American Males with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Reduces Migraines in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin's Impact on Joint Health in American Males with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin: Enhancing Skin Elasticity and Well-being in Men with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Enhances Memory in American Men with Growth Hormone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Norditropin Therapy: Enhancing Muscle Health in American Males with GHD [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin's Potential in Reducing Depression in American Males with Growth Hormone Deficiency [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin's Impact on Cardiovascular Health in American Men with GHD [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin: Managing Growth Hormone Deficiency with Gastrointestinal Disorders [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Norditropin Therapy Enhances Throat Health in American Males with GHD [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Norditropin Enhances Eye Health in Growth Hormone Deficient American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Norditropin's Potential in Reducing Sinus Issues for American Males with GHD [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Norditropin's Impact on Anemia in American Males with Growth Hormone Deficiency [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Norditropin Therapy Enhances Vascular Health in American Males with GHD [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Norditropin's Impact on Lymphatic Health in American Males with Growth Hormone Deficiency [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Norditropin Enhances Blood Health in American Men with Growth Hormone Deficiency [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Norditropin: Enhancing Endocrine Health in American Males with Growth Hormone Deficiency [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Norditropin's Potential in Treating GHD and Autoimmune Diseases in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin's Impact on Nervous System Health in Growth Hormone Deficient American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin's Efficacy in American Males with GHD and Allergic Disorders [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Norditropin Therapy Enhances Immune Function in American Males with GHD [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Norditropin Therapy Enhances Reproductive Health in American Males with GHD [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Norditropin Therapy Enhances Musculoskeletal Health in American Males with GHD [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Norditropin: Enhancing Gastrointestinal Health in American Males with Growth Hormone Deficiency [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Norditropin Enhances Digestive Health in American Males with Growth Hormone Deficiency [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Norditropin's Effects on Urinary Health in American Males with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Norditropin Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Norditropin's Efficacy in Treating GHD and Skin Disorders in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Norditropin: Enhancing Hair Health in Men with Growth Hormone Deficiency [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Norditropin's Potential in Reducing Sinus Issues in American Males with GHD [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Norditropin Therapy's Impact on Throat Health in American Males with GHD [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Norditropin Therapy Enhances Nail Health in American Men with Growth Hormone Deficiency [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]