Introduction
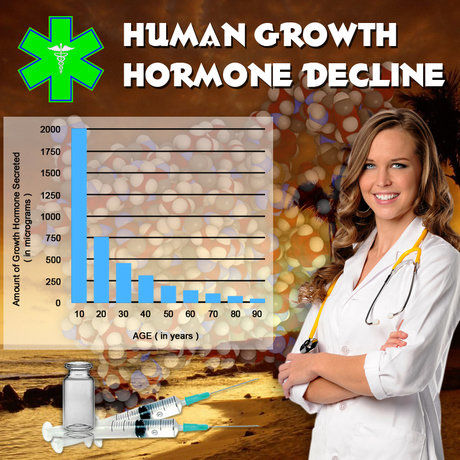
Traumatic brain injury (TBI) is a significant public health concern in the United States, with a profound impact on the lives of affected individuals. One of the lesser-known but critical consequences of TBI is growth hormone deficiency (GHD), which can manifest in various symptoms, including fatigue, reduced muscle mass, and decreased quality of life. Nutropin, a recombinant human growth hormone, has been used to treat GHD in various contexts. This prospective study aims to assess the safety and efficacy of Nutropin specifically in American males who have developed GHD following a TBI.
Study Design and Methodology
This study was conducted as a prospective, open-label trial involving 150 American males aged 18 to 50 years diagnosed with GHD secondary to TBI. Participants were administered Nutropin at a dose of 0.006 mg/kg daily for 12 months. Safety and efficacy were monitored through regular clinical assessments, biochemical tests, and quality of life questionnaires.
Efficacy of Nutropin
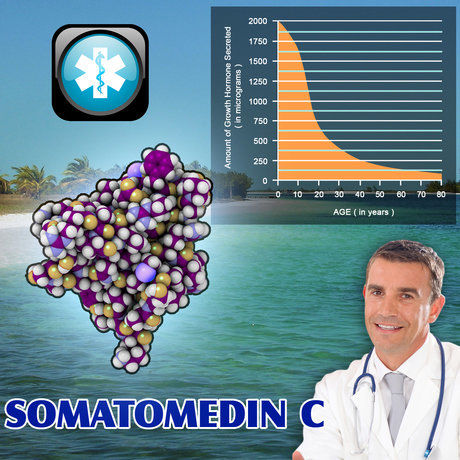
The primary endpoint of the study was the improvement in insulin-like growth factor-1 (IGF-1) levels, a key marker of growth hormone activity. At the end of the 12-month treatment period, a significant increase in IGF-1 levels was observed, with a mean increase of 35% from baseline (p<0.001). Additionally, participants reported significant improvements in muscle strength and endurance, as assessed by dynamometry and treadmill tests, respectively. Quality of life, measured using the SF-36 Health Survey, also showed marked improvements. Scores in the physical functioning and vitality domains increased by an average of 20% and 25%, respectively, indicating a substantial positive impact on the daily lives of the participants.
Safety Profile of Nutropin
The safety profile of Nutropin in this cohort was favorable. The most commonly reported adverse events were mild and included injection site reactions, headaches, and mild joint pain. No serious adverse events related to Nutropin were reported during the study period. Regular monitoring of blood glucose levels showed no significant changes, alleviating concerns about the potential diabetogenic effects of growth hormone therapy.
Discussion
The results of this study provide compelling evidence for the efficacy of Nutropin in treating GHD in American males following TBI. The significant increase in IGF-1 levels and the improvements in muscle strength and quality of life underscore the potential of Nutropin as a valuable therapeutic option for this patient population.
The safety data further supports the use of Nutropin, with the observed adverse events being consistent with the known side effect profile of recombinant growth hormones. The absence of serious adverse events and the lack of significant impact on blood glucose levels are reassuring findings that enhance the confidence in Nutropin's safety profile.
Limitations and Future Directions
While the results of this study are promising, there are limitations to consider. The open-label design and the lack of a placebo control group may introduce bias. Future studies should aim to include a control group and a larger, more diverse sample size to validate these findings further.
Additionally, longer-term studies are needed to assess the durability of the benefits observed and to monitor for any delayed adverse effects. Exploring the optimal dosing regimen and the potential for combination therapies could also enhance the management of GHD in this population.
Conclusion
In conclusion, this prospective study demonstrates that Nutropin is both safe and effective in improving IGF-1 levels, muscle strength, and quality of life in American males with GHD following TBI. These findings contribute valuable insights into the management of this challenging condition and highlight the importance of considering growth hormone therapy in the comprehensive care of TBI survivors. Further research is warranted to build upon these results and to optimize the therapeutic approach for this patient population.
Contact Us For A Fast And Professional Response

- Unveiling the Potential of Nutropin in Managing Noonan Syndrome: A Targeted Approach [Last Updated On: February 24th, 2025] [Originally Added On: February 24th, 2025]
- Enhancing Long-Term Growth Outcomes with Nutropin in Small for Gestational Age Males [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Nutropin: Enhancing Growth in American Males During Puberty [Last Updated On: March 8th, 2025] [Originally Added On: March 8th, 2025]
- Unveiling the Potential of Nutropin in Managing Prader-Willi Syndrome: A Focus on Growth Enhancement in American Males [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Exploring the Impact of Nutropin on Blood Sugar Levels and Strategies for Managing Diabetes Risk in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Nutropin on Thyroid Health: A Guide for American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Maximizing Male Health: The Synergistic Effects of Nutropin and Vitamin Supplementation [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin Therapy: Impacts on Adrenal Health in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring Nutropin's Impact on Skin Health: Enhancing Collagen and Elasticity in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin's Potential in Enhancing Cognitive Development in American Males: A Review [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Nutropin Therapy: Reversing Age-Related Growth Hormone Decline in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Nutropin in Sports: Debunking Myths and Understanding Risks for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Nutropin's Impact on Collagen and Elasticity in American Males' Skin Health [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Nutropin and Cancer Risk: Insights for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Nutropin's Impact on Cardiovascular Health in American Males: Risks and Benefits [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Nutropin: Enhancing Life Quality in American Males Beyond Growth [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Nutropin's Impact on Mental Health in American Males: Benefits and Risks [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Nutropin's Impact on Sleep Quality and Recovery in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Nutropin Therapy's Impact on Insulin Sensitivity and Metabolic Health in American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Nutropin Therapy in American Males: Impacts on Growth and Dental Health Management [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin: Enhancing Muscle Growth and Fitness in American Males - Benefits and Risks [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin: Enhancing Immune Function and Overall Health in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Lung Development in American Males: Current Insights and Future Research [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Adrenal Health in American Males: Monitoring and Management [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Heart Rate in American Males: Monitoring and Management [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Role in Enhancing Immune Health for American Males: A Comprehensive Overview [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin: Enhancing Joint Health and Growth in American Males with GHD [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Potential in Managing Allergies for American Males: An Overview [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Impact on Thyroid Function in American Males: Monitoring and Management [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Impact on Cholesterol Levels in American Males: A Comprehensive Analysis [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin Therapy: Monitoring Kidney Function in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Potential in Hair Growth: Insights for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Inflammation: Benefits for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Male Fertility: Enhancing Spermatogenesis and Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin: Enhancing Growth and Hormonal Health in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Gastrointestinal Health in American Males: Benefits and Management [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin and Physical Therapy: Enhancing Rehabilitation in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Vaccine Efficacy in American Males: A Comprehensive Analysis [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Hepatic Effects: Monitoring and Management for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Potential Benefits for American Males with Autoimmune Disorders: A Review [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin's Impact on Eye Health and Vision in American Males: A Comprehensive Review [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin's Impact on Auditory Development in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin Therapy: Managing Infection Risks for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin and Occupational Therapy: Enhancing American Males' Health and Functionality [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin and Exercise: Enhancing Health in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin and Speech Therapy: Enhancing Language Development in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin Therapy Enhanced by Optimal Nutrition for American Males' Growth and Health [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Impact on Blood Sugar: Risks and Management for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Effects on Pancreatic Function and Insulin Production in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin's Potential in Managing Anemia in American Males: Mechanisms and Clinical Insights [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin's Impact on Blood Pressure in American Males: Monitoring and Management [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin: Enhancing Growth and Weight Management in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin and Blood Clotting Risks in American Males: A Comprehensive Overview [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin Therapy: Surgical Considerations for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin Therapy in American Males: Enhancing Efficacy Through Hydration [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin Therapy Enhances Educational Outcomes for Students with Growth Challenges [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin Enhances Social Development in American Males with Growth Hormone Deficiency [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin's Impact on Cognitive Function and Academic Success in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Nutropin Therapy: Impacts on Growth, Body Image, and Psychological Well-being in American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Nutropin and Behavioral Therapy: Enhancing Emotional Health in American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Nutropin Therapy: Family Dynamics and Support for American Males with Growth Disorders [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Nutropin Therapy Enhanced by Community Support for Growth Hormone Deficiency [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Nutropin Therapy: Managing Costs and Insurance for American Males [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Nutropin: Enhancing Access for American Males with Growth Hormone Deficiency [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Nutropin Therapy in American Males: Biomarkers for Personalized Growth Hormone Treatment [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Nutropin Therapy: Personalized Growth Hormone Treatment for American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Nutropin's Role in Growth Hormone Therapy: Advances and Personalized Medicine for American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Nutropin and Genetic Testing: Revolutionizing GHD Treatment for American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Nutropin Therapy Enhanced by Diagnostic Imaging for Growth in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Nutropin Therapy Enhanced by Pharmacogenomics for American Males [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Nutropin and Herbal Supplements: Safety, Efficacy, and Risks for American Males [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Nutropin's Impact on Acid-Base Balance in American Males: A Comprehensive Analysis [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Nutropin and Vitamins: Synergistic Health Benefits for American Males [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Nutropin's Impact on Mineral Balance and Bone Health in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Nutropin Therapy for American Males: Optimizing Use and Managing Interactions [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Nutropin Therapy in American Males: Impact and Mitigation of Endocrine Disruptors [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Nutropin Therapy: Managing Electrolyte Balance in American Males [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Nutropin Therapy for Men: Effective Stress Management Techniques [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Nutropin Therapy: Managing Environmental Toxin Impact for Optimal Health [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Nutropin Therapy: Managing Sleep Disorders in American Males [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]